University Hospitals Coventry and Warwickshire NHS Trust
CASE STUDY: University Hospitals Coventry and Warwickshire (UHCW) NHS Trust
One-time winner for trust’s future-proof endoscope reprocessing scheme
Following a competitive evaluation based on an eight-year life cycle for endoscope reprocessing equipment the choice was Steelco, with its One-time Connection System.

"The cost saving with iM Med handling the servicing is substantial"
Following a competitive evaluation based on an eight-year life cycle for endoscope reprocessing equipment the choice was Steelco, with its One-time Connection System.
The scheme, for University Hospitals Coventry and Warwickshire (UHCW) NHS Trust, has been managed by Steelco’s UK distributor, iM Med.
“iM Med were already providing the service and maintenance support and their own type tested chemistry to keep the old endoscope washer-disinfectors (EWDs) going,” explains Simon Lambert, UHCW’s decontamination lead. “The machines had reached the end of their life and iM Med’s work gave us the breathing space to look at what we required.”
When they took over the services and maintenance contract, iM Med had given a ‘health check’ to each of the old EWDs and the drying and storage cabinets.”
Simon says: “The cost saving with iM Med handing the servicing is substantial. The main benefit was the provision and ready availability of a fully trained engineer, who brought in any parts that were required. The chemistry is cost-effective too.
“We have regular KPI meetings, which are a benefit. It’s good to understand the level of call outs and to have a comparison against the previous service supplier. iM Med’s engineer response time is impressive and the work is of a good standard.”
"We chose Steelco because of the usability. It’s a user-friendly system, with a one-time connection throughout the process - pre-washing, washing and decontamination, and drying and storage. We felt that one-time handing was the main advantage."
In the ‘breathing space’, the trust was looking at a plan to replace the aged endoscope reprocessing equipment at University Hospital, Coventry and considering new options, including centralisation. There was also a desire to invest in endoscopy at the trust’s Hospital of St Cross, in Rugby.
“Working with the trust’s AE(D), Jim Tinsdeall, we did an evaluation,” says Simon. “We wrote a specification and looked for the equipment to cover it. Chemistry, consumables and engineering - the quality of the build and reliability - were important factors.
“I wasn’t initially aware that iM Med is Steelco’s UK distributor. It’s known that Steelco machines are very reliable and effective. We were able to visit a London hospital to see their Steelco installation and to talk to the management and users there about their experiences with the equipment.”
Simon explains: “We chose Steelco because of the usability. It’s a user-friendly system, with a one-time connection throughout the process - pre-washing, washing and decontamination, and drying and storage. We felt that one-time handing was the main advantage.
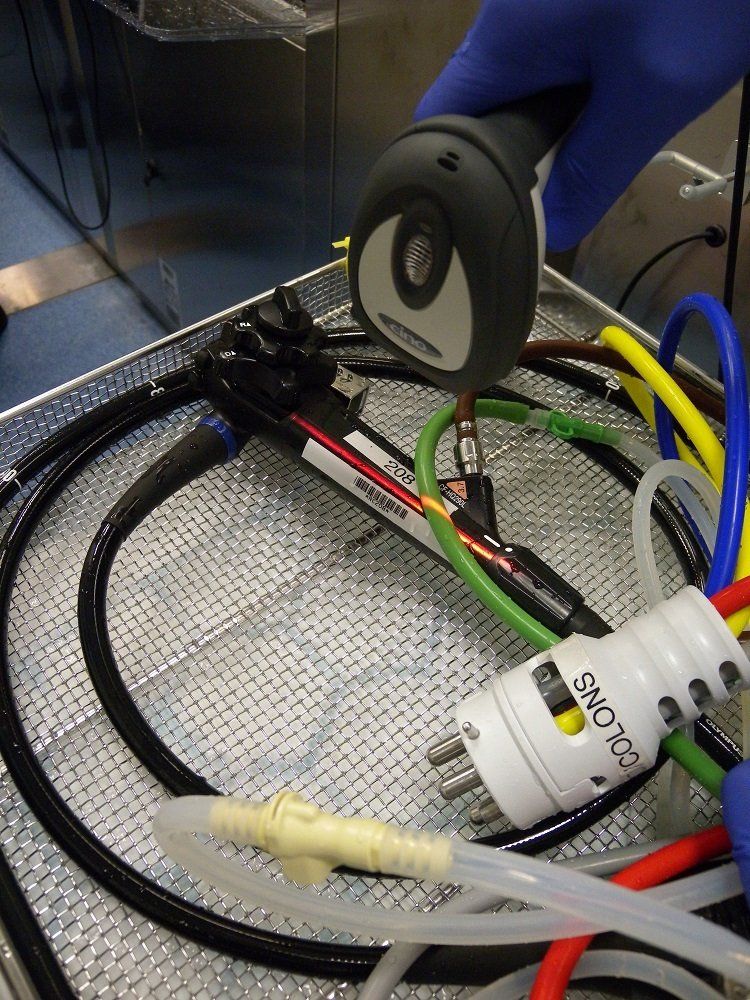
“The scope goes from the pre-clean connected to a hub straight into the EWD, then transferred to a drying and storage cabinet using the same connector. The cost of the chemistry was an important factor too.
“The Steelco offering has a lot of choice with the type of machine. They have two and three-scope chamber machines, with the same footprint, so we could achieve more throughput in the fairly limited space we have. We could tailor the selection to suit our requirements and build in capacity for future-planning.
“I prepared a ‘Case for Replacement’ and presented it to the capital planning group,” says Simon. “It was a trust funded scheme, which we bought through NHS Supply Chain. They do a lot of the hard work for you. There was a discount available from Steelco too, so there was a financial benefit as well.”
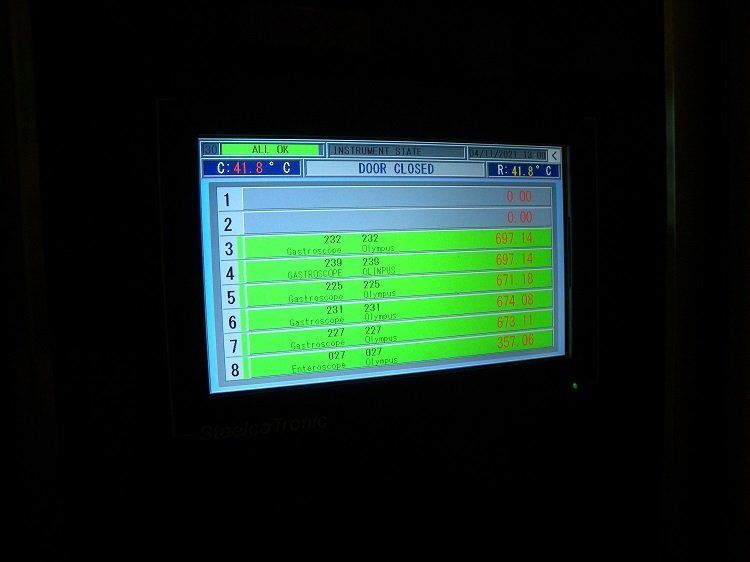
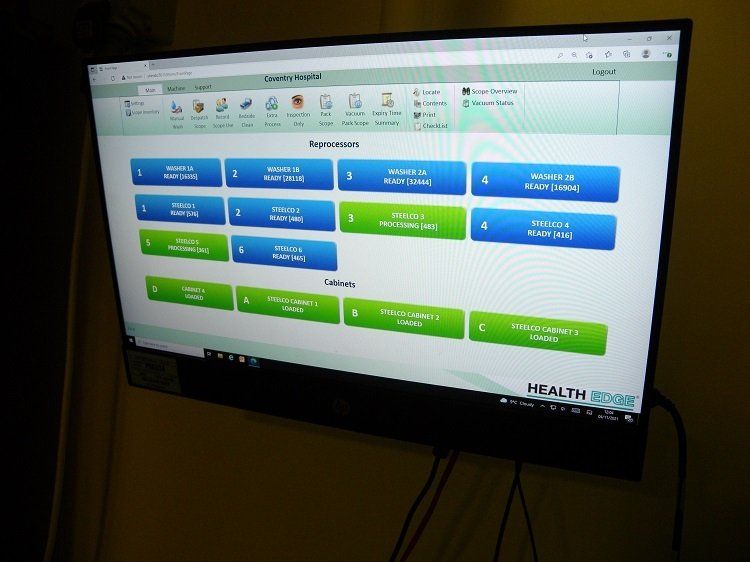
The UHCW programme, which is now completed, includes the installation of Steelco’s automated system to assist manual cleaning, four three-scope EWDs and one two-scope EWD in the main endoscopy department, together with five drying and storage cabinets.
As the theatres at the trust have a small footprint, a decision was taken to reposition the department’s endoscope reprocessing into a quality environment within sterile services.
Both endoscopy and sterile services have iM Med’s EndoPax vacuum packing system for the dry storage and transportation of reprocessed scopes. iM Med also supplies the trust with a range of cost-effective endoscope consumables, including transport bags, chemistry for the manual wash, connectors, O rings and other ancillary items.
After the requirement for a peracetic acid monitoring system was highlighted in the JAG IHEEM audit, a ChemDAQ system - distributed in the UK by iM Med - was installed in endoscopy. This provides assurance of staff safety, together with continuous monitoring and recording, giving tracking for JAG monthly reports, which can be time-weighted and downloaded.
The plan for Hospital of St Cross, in Rugby, will see an expansion of endoscopy with additional procedure rooms and the installation of three Steelco EWDs - two three-scope machines and one two-scope machine.
“We continued to supply a full service while all the work took place,” explains Simon. “The removal of the old machines and installation of the new Steelco EWDs and drying cabinets was well coordinated by the staff and the departments and by iM Med engineers, Andrew Thomas and Paul Moseley.
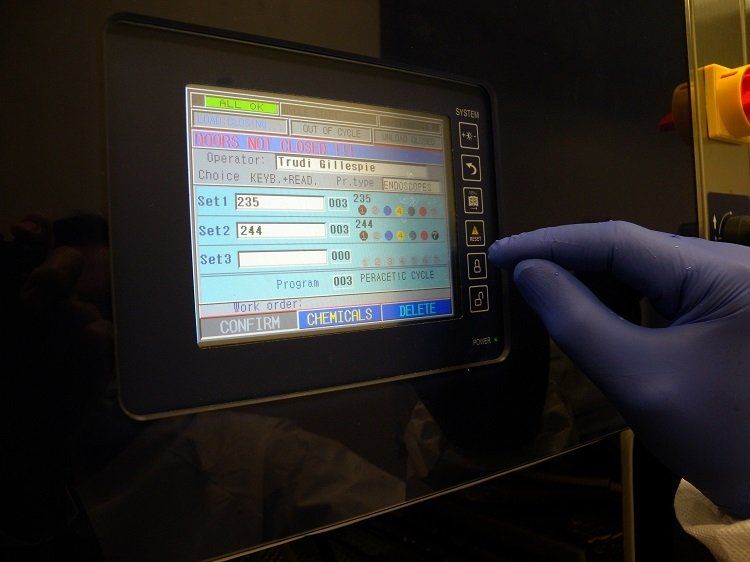
“We set up a project team from the start. It included a trust team, iM Med and the trust’s PFI partner, Vinci. Joe Colby, lead nurse and endoscopy unit manager, Stuart Brooks, SSD quality manager, Neil Harper, SSD manager, Trudi Gelipse and Kay Harper, endoscopy technicians, played key roles in supporting the project.
“All the machines were installed and commissioned, and validation reports completed. iM Med’s Karen McHale delivered a full training package to the technicians.”
With the programme now completed, the trust’s AE(D), Jim Tinsdeall, says: “The trust was already getting a good service from iM Med on the old endoscope washer-disinfectors. It was a competitive tender for the new machines, based on cost and quality. The cost element covered the eight-year life cycle of the machines - including capital cost, maintenance and validations, and the cost of the chemistry. The iM Med chemistry is type tested and has a competitive price.
“The installation went well and the validations met all our requirements and, indeed, iM Med exceeded our expectations with some of the validations.”
Jim adds: “I recommended a peracetic acid monitoring system in my JAG IHEEM audit. Organisations have options in how to meet the requirement. They can get a company in to undertake some occupational monitoring, which does not give you a constant record of the situation, or they can install a continuous monitoring system. I was happy that iM Med had a system available.”
Describing in more detail how the installation programme ran, Andrew Thomas, iM Med’s technical service manager, explains: “We started the service and maintenance contract on the old machines at UHCW in 2018, so we had plenty of experience in how the department operated and knew the relatively small footprint that was available.
“At the start of the tender process we gave the trust several options and configurations that suited the layout of the endoscopy department. The L-shape of the reprocessing area, had washer-disinfectors along both walls. This allowed us to create a good segregation between four of the old machines on one wall, and the work side, where we removed the remainder of the old machines and installed the higher capacity, new machines.
“The remaining four old machines continued to service the department during the installation, while we carried out the installation behind a temporary screen.
“We drew up a project plan, including a Gantt chart of the tasks, timelines, etc. There were weekly project planning meetings between ourselves, the trust and Vinci, the PFI company.”
Gantt charts are used for planning and scheduling projects in project management. A Gantt chart allows you to simplify complex projects into an easy-to-follow plan and track the status of tasks as work progresses.
Andrew says: “The reverse osmosis system was handled by the trust, while most of the enabling works, the electrics and water, were done by the PFI contractor. iM Med undertook a limited level of adaptions, largely covering the drainage adjustments and updates. We worked with the RO company to ensure there were no intrusions with their pipework and that the points of use were in the correct positions for the new machines.
“Coordination went well, thanks to the weekly meetings, and everything stayed on track, on schedule and on time.
“When it came to the heavy work, the first part was to remove three of the old EWDs,” says Andrew. “That was done over Easter weekend, 2021, while the department was closed. We left in two of the old two-chamber machines to enable the department to continue to operate. The old drying and storage cabinets were relocated to a nearby, temporary location so they could continue to be used.
“That weekend we put up some temporary screens to allow the PFI company to install some services overhead, ready for the installation of the new machines.
Then, over a couple of evenings, out of hours, we were able to move the new washer-disinfectors into position. We always try to handle all the disruptive work out of hours.
“The installation, commissioning and validation of the bank of new machines and the drying and storage cabinets took a few weeks. Being segregated, it was like a fresh installation, without causing any interference to the existing area, which was able to remain operational throughout. It all went well. We have quite a lot of procedures and protocols in place to ensure projects run smoothly and to time.”
Andrew explains: “All iM Med’s engineers have undertaken factory engineer training on the range of Steelco endoscopy equipment. We’ve now completed quite a number of installations similar to the one at Walsgrave Hospital and they’ve all gone smoothly.”
Steelco’s EWDs have vertical sliding doors which will allow the technicians more room to operate. As the new machines are more efficient and have shorter cycle times, it was only necessary to install them on one wall of the L-shape. This has created 50% more space in the area, which will be used as packing space, etc giving the users a more comfortable working environment.
Andrew says: “We’re very conversant with the old washer-disinfectors that have been replaced and understand all the issues in terms of decommissioning. We can handle that in-house. So, there’s no need for a third party to be involved. From the customer’s point of view, they are only dealing with one company.
“Once the new machines were handed over our dedicated engineer, Paul Moseley, was on site to ensure everything went smoothly and that there are no teething problems for the staff. From the ‘Go Live’ date the team had the reassurance of the old machines being there as a safety blanket. However, we didn’t envisage any issues. After a couple of weeks of running the new machines, and when the team are fully conversant with them, we will remove the remining four old machines and the installation of the ‘new-style’ department will be complete.
“The move to reprocess scopes from theatres in sterile services is a change for the department. It’s on a different timescale to endoscopy. The building and enabling work were done earlier and the EWD has only recently been installed. It’s a pass-through machine, with the drying and storage cabinet situated in the clean room. This is how we’ve handled similar installations where trusts have moved to a more centralised facility.
“Throughout the process we kept in close contact with Jim Tinsdeall, the AE(D),” adds Andrew. “Commissioning went well, and all the validation documents were submitted to the AE(D). It was a good collaboration between ourselves, the trust and the PFI contractor. Everybody was on board and fully supportive to get the scheme completed, in particular Joe Colby in endoscopy and Neil Harper, in sterile services.”
With the scheme now completed, Simon Lambert adds: “The concept of One-time connection and compatibility across the three areas - main endoscopy, sterile services and Hospital of St Cross - is key, both in terms of contingency and the support of staff if required. We’ve created more space in the departments and through using multi-chamber machines we’ve reduced the life costs of the equipment as we’re using less chemistry.
“It’s a credit to all the team that this was achieved without any disruption to the normal service. iM Med’s support and service throughout was excellent.
I’ll be looking to add a Steelco ultrasonic machine in the future to replace our old unit. The Steelco range covers all aspects of decontamination, even including low temperature hydrogen peroxide sterilisation which we will be considering.”
For further information, call iM Med on 01223 440 475
THE DECONTAMINATION SPECIALISTS
Get the latest decontamination news and updates
Contact Us
Thank you for contacting us.
We will get back to you as soon as possible
We will get back to you as soon as possible
Oops, there was an error sending your message.
Please try again later
Please try again later